What does a positive value of G mean for a reaction?
A. The reaction is non spontaneous.
B. T...

Chemistry, 16.07.2021 23:20, savid88061
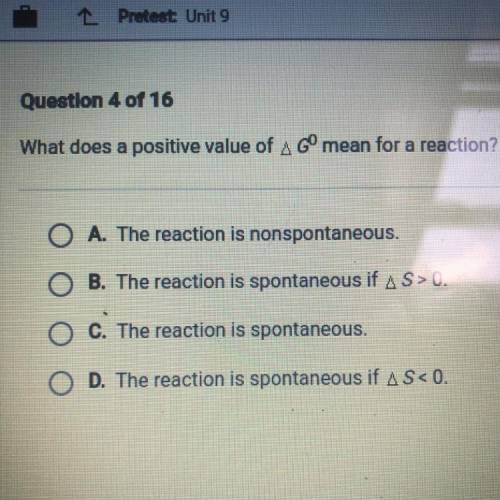
What does a positive value of G mean for a reaction?
A. The reaction is non spontaneous.
B. The reaction is spontaneous if S>0.
C. The reaction is spontaneous.
D. The reaction is spontaneous if S<0.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, pup88
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 23:30, ninilizovtskt
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2

Chemistry, 23.06.2019 03:00, yoongislaugh
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3
Do you know the correct answer?
Questions in other subjects:

Biology, 11.11.2020 05:50

Mathematics, 11.11.2020 05:50


English, 11.11.2020 05:50

Mathematics, 11.11.2020 05:50


Mathematics, 11.11.2020 05:50


Mathematics, 11.11.2020 06:00

Mathematics, 11.11.2020 06:00






