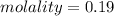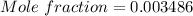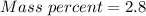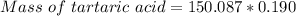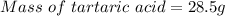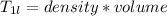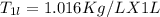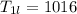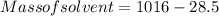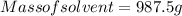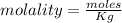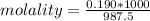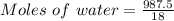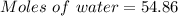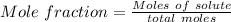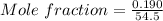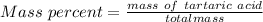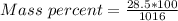Chemistry, 16.07.2021 01:00, 911wgarcia
A beverage contains tartaric acid, H2C4H4O6, a substance obtained from grapes during wine making. If the beverage is 0.190 tartaric acid, what is the molal concentration? What is the mole fraction of tartaric acid and water? Calculate the mass percent of tartaric acid. The density of the solution is 1.016g/mL.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, ian2006huang
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3


Chemistry, 22.06.2019 19:30, Karinaccccc
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 23.06.2019 05:00, MoneyMike42
Select the statement that describe chemical properties a. antacid tablets neutralize stomach acid b. helium is the lightest monatomic element c. water freezes at 0 celsius d. mercury is liquid at room temperature
Answers: 3
Do you know the correct answer?
A beverage contains tartaric acid, H2C4H4O6, a substance obtained from grapes during wine making. If...
Questions in other subjects:



History, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00



Mathematics, 18.03.2021 03:00

Social Studies, 18.03.2021 03:00