Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, ayaanwaseem
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 12:40, jaylen2559
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 23.06.2019 00:30, mathwiznot45
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1

Do you know the correct answer?
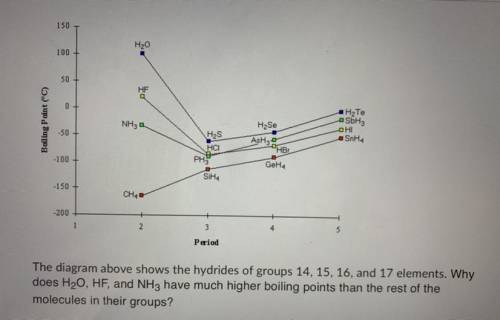
The diagram above shows the hydrides of groups 14, 15, 16, and 17 elements. Why does H20, HF, and NH...
Questions in other subjects:


Mathematics, 21.04.2020 01:47