
Chemistry, 15.07.2021 14:50, triciajfive
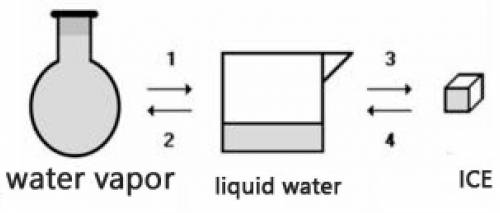
Consider the transformations a water sample undergoes without external pressure variation
(a) Transformations 2 and 4 are endothermic.
(b) Transformations 1 and 2 are exothermic.
(c) The amount of energy absorbed in 3 is equal to the amount released in 1.
(d) The amount of energy released in 2 is equal to the amount released in 4.
(e) The change of physical state does not involve heat energy.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, yaneiryx5476
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 14:30, isaiahrodriguezsm17
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 14:50, chem1014
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2
Do you know the correct answer?
Consider the transformations a water sample undergoes without external pressure variation
(a) Trans...
Questions in other subjects:



History, 09.07.2021 19:50


Chemistry, 09.07.2021 20:00




English, 09.07.2021 20:00

Mathematics, 09.07.2021 20:00






