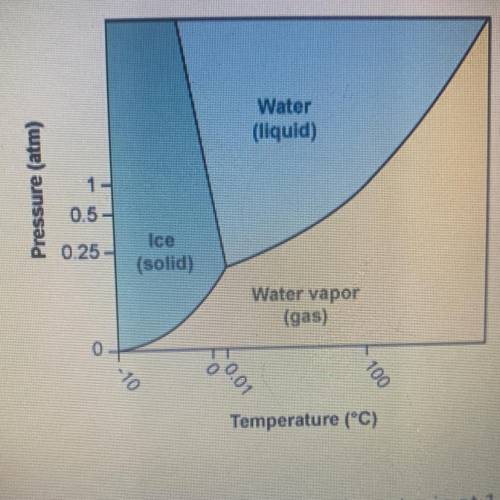
Using the phase diagram for H20, what phase is water in at 1 atm pressure
and -5°C?
A. It is...


Answers: 3
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:00, samangelzrose3576
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1

Chemistry, 23.06.2019 10:30, dreamxette3119
Fill in the blanks for the following statements: the rms speed of the molecules in a sample of h2 gas at 300 k will be times larger than the rms speed of o2 molecules at the same temperature, and the ratio µrms (h2) / µrms (o2) with increasing temperature. a not enough information is given to answer this question b sixteen, will not change c four, will not change d four, will increase e sixteen, will decrease
Answers: 2


Chemistry, 23.06.2019 13:30, Catracho3619
32p and 31p are two isotopes of phosphorus. compare the number if subatomic particles that are present in the atoms of these isotopes.
Answers: 1
Do you know the correct answer?
Questions in other subjects:





Biology, 04.05.2021 03:00

History, 04.05.2021 03:00

Chemistry, 04.05.2021 03:00

Spanish, 04.05.2021 03:00

Mathematics, 04.05.2021 03:00