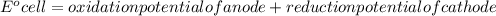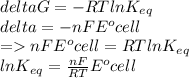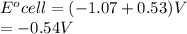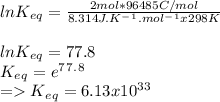Chemistry, 14.07.2021 20:10, SkyeShadow525
Determine the equilibrium constant, Keq, at 25°C for the reaction
2Br- (aq) + I2(s) <--> Br2(l) + 2I- (aq)
Eocell = (0.0257/n) lnKeq, Calculate Eocell from Use this equation to calculate K value.
Eo (I2/I-) = +0.53, Eo (Br2/Br-) = +1.07,

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, vannitling12p4w44f
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 21:00, lalaween098
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3


Chemistry, 23.06.2019 09:30, crystalhoff9419
My plate recommends that of your nutritional intake comes from fruits and vegetables. a. 30% b. 50% c. 20% d. 40%
Answers: 2
Do you know the correct answer?
Determine the equilibrium constant, Keq, at 25°C for the reaction
2Br- (aq) + I2(s) <--> Br2(...
Questions in other subjects:

English, 17.10.2019 17:30

Mathematics, 17.10.2019 17:30





Mathematics, 17.10.2019 17:30

Mathematics, 17.10.2019 17:30

Mathematics, 17.10.2019 17:30

Mathematics, 17.10.2019 17:30