
Chemistry, 13.07.2021 16:30, aesthetickait
The following pairs of soluble solutions can be mixed. In some cases, this leads to the formation of an insoluble precipitate. Decide, in each case, whether or not an insoluble precipitate is formed.
a. K2S and NH4Cl
b. CaCl2 and NH4CO3
c. Li2S and MnBr2
d. Ba(NO3)2 and Ag2SO4
e. RbCO3 and NaCl

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:00, bbrogle5154
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 19:00, georgesarkes12
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 22.06.2019 21:40, k3rbycalilung
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2

Chemistry, 22.06.2019 23:00, Mynameismath
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3
Do you know the correct answer?
The following pairs of soluble solutions can be mixed. In some cases, this leads to the formation of...
Questions in other subjects:


English, 21.12.2020 07:50


Mathematics, 21.12.2020 07:50


Mathematics, 21.12.2020 07:50




History, 21.12.2020 07:50


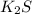 and
and 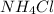 :
: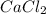 and
and 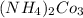 :
: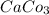 forms.
forms.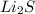 and
and 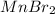 :
: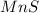 forms.
forms.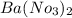 and
and 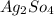 :
: 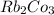 and
and 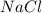 :
: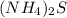 ⇒ soluble.
⇒ soluble. ⇒ soluble,
⇒ soluble, 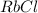 ⇒ soluble,
⇒ soluble, 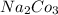 ⇒ soluble.
⇒ soluble.



