
Octane (C8H18) is a component of gasoline that burns according to the following equation: C8H18(l) + 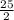 O2(g) → 8CO2(g) + 9H2O(g) ΔH∘rxn = −5074.1 kJ
What mass of octane (in g) is required to produce 8210 kJ of heat?
O2(g) → 8CO2(g) + 9H2O(g) ΔH∘rxn = −5074.1 kJ
What mass of octane (in g) is required to produce 8210 kJ of heat?
Express your answer to three significant figures.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, dinosaur10
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 06:30, irvinbhangal2
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 07:40, sadcase85
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na, so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1
Do you know the correct answer?
Octane (C8H18) is a component of gasoline that burns according to the following equation: C8H18(l) +...
Questions in other subjects:





Mathematics, 28.07.2020 23:01

Mathematics, 28.07.2020 23:01

Mathematics, 28.07.2020 23:01

Mathematics, 28.07.2020 23:01


Social Studies, 28.07.2020 23:01






