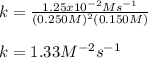NO2 (g) reacts with O2 4 NO2 (g) + O2(g) to produce N2 O5 (g) as shown by the equation below: (g) = 2 N2 O5 (g).
Experimentally the rate orders were determined and rate law written as shown below: Rate = k [NO2]2[O2 ].
Calculate the value of k if the initial concentration of NO2 was 0.250 M and initial
concentration of O2 (g) was 0.150 M. The initial rate was 1.25 x 10-2 M. s-1 .

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, SilverTheAmarok
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 10:30, Wookas8355
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 16:50, TrueKing184
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1
Do you know the correct answer?
NO2 (g) reacts with O2 4 NO2 (g) + O2(g) to produce N2 O5 (g) as shown by the equation below: (g) =...
Questions in other subjects:




Mathematics, 25.02.2021 23:50


Biology, 25.02.2021 23:50

Spanish, 25.02.2021 23:50

Spanish, 25.02.2021 23:50


History, 25.02.2021 23:50


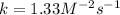
![k=\frac{r}{[NO_2]^2[O_2]}](/tpl/images/1392/3789/849e7.png)