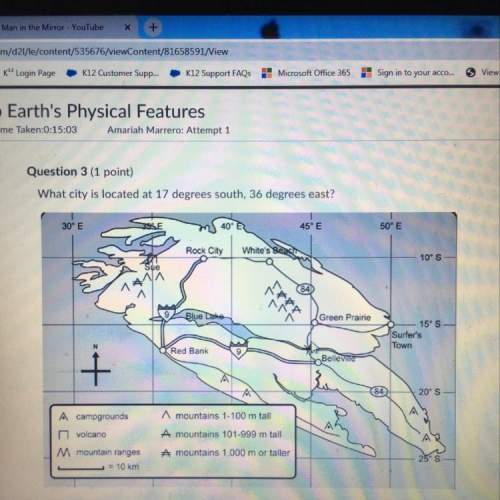
Chemistry, 10.07.2021 05:40, ayshearouse1203
Butane (C4H10(9), AH = -125.6 kJ/mo) reacts with oxygen to produce carbon dioxide (CO2. AH+ = -393.5 kJ/mol) and
water (H20, AH+ = -241.82 kJ/mol) according to the equation below.
2C4H10(g) + 1302(g) → 8C02(g) + 10H2O(g)
What is the enthalpy of combustion (per mole) of C4H10(9)?
Use AH yen-(AHproducts) - ( He reactants)
-2,657.5 kJ/mol
0-5315.0 kJ/mol
--509.7 kJ/mol
O-254.8 kJ/mol

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:50, nnaomii
What is the composition, in atom percent, of an alloy that consists of 4.5 wt% pb and 95.5 wt% sn? the atomic weights for pb and sn are 207.19 g/mol and 118.71 g/mol, respectively.(a) 2.6 at% pb and 97.4 at% sn(b) 7.6 at% pb and 92.4 at% sn(c)97.4 at% pb and 2.6 at% sn(d) 92.4 at% pb and 7.6 at% sn
Answers: 2

Chemistry, 21.06.2019 22:30, monnn91351
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2


Chemistry, 22.06.2019 19:30, xxaurorabluexx
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
Do you know the correct answer?
Butane (C4H10(9), AH = -125.6 kJ/mo) reacts with oxygen to produce carbon dioxide (CO2. AH+ = -393.5...
Questions in other subjects:


French, 25.02.2021 15:00

English, 25.02.2021 15:00


History, 25.02.2021 15:00




Mathematics, 25.02.2021 15:00

Mathematics, 25.02.2021 15:00