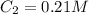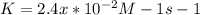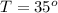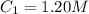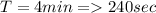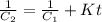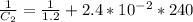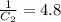Answers: 2
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 09:00, payshencec21
How many grams of ammonia are produced when 1.0 mole of nitrogen reacts
Answers: 2

Do you know the correct answer?
The rate constant for a particular second order reaction is 2.4 x 10-2 M-1 s-1 at 35∘C. If the initi...
Questions in other subjects:


Mathematics, 07.05.2021 01:30

Mathematics, 07.05.2021 01:30



Mathematics, 07.05.2021 01:30


Business, 07.05.2021 01:30