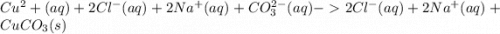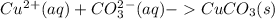Chemistry, 10.07.2021 02:30, stgitskaysie9028
g When copper(II) chloride and sodium carbonate solutions are combined, solid copper(II) carbonate precipitates, leaving a solution of sodium chloride. Write the conventional equation, total ionic equation, and net ionic equation for this reaction. (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:20, Jessicadiaz8602
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 17:30, nijanicole164
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 22:30, angelagonzalesownus1
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Do you know the correct answer?
g When copper(II) chloride and sodium carbonate solutions are combined, solid copper(II) carbonate p...
Questions in other subjects:


Mathematics, 04.05.2021 03:20


Chemistry, 04.05.2021 03:20

History, 04.05.2021 03:20

Mathematics, 04.05.2021 03:20



Mathematics, 04.05.2021 03:20

Mathematics, 04.05.2021 03:20