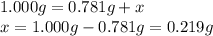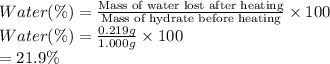Chemistry, 08.07.2021 18:40, brianmondesir1owahud
This question has multiple parts.
Part A:
A sample of unknown hydrate, AC-XH20, has a mass of 1.000 g before heating and a
mass of 0.781 g after heating. What is the experimental percentage of water in this
unknown hydrate?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, KnMcdonaldk93906
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1


Chemistry, 21.06.2019 23:00, cami30031cami3003
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 07:00, haydjanggg6578
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2
Do you know the correct answer?
This question has multiple parts.
Part A:
A sample of unknown hydrate, AC-XH20, has a mass of...
A sample of unknown hydrate, AC-XH20, has a mass of...
Questions in other subjects:

Mathematics, 21.01.2021 21:00


Spanish, 21.01.2021 21:00





Social Studies, 21.01.2021 21:00

Mathematics, 21.01.2021 21:00

Mathematics, 21.01.2021 21:00