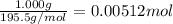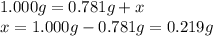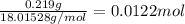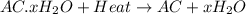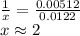A sample of unknown hydrate, AC-XH20, has a mass of 1.000 g before heating and a
mass of 0.781 g after heating. If the molar mass of the anhydrous compound (AC) is
195.5 g/mol, what is the water of crystallization for the formula of the unknown
hydrate?
Type your work for partial credit.
Answer choices: 2, 3, 5, or 6.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, alaina3792
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2


Chemistry, 22.06.2019 10:00, micahwilkerson9495
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2
Do you know the correct answer?
A sample of unknown hydrate, AC-XH20, has a mass of 1.000 g before heating and a
mass of 0.781 g af...
Questions in other subjects:


Physics, 09.06.2021 08:10

Mathematics, 09.06.2021 08:10

Mathematics, 09.06.2021 08:10

Mathematics, 09.06.2021 08:10

Mathematics, 09.06.2021 08:10

Biology, 09.06.2021 08:10


Mathematics, 09.06.2021 08:10

Advanced Placement (AP), 09.06.2021 08:10