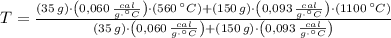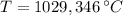Chemistry, 08.07.2021 06:20, kyliefetters11
Para formar bronce, se mezclan 150g de cobre a 1100°C y 35g de estaño a 560°C. Determine la temperatura final del sistema.
Dato: Ce Cu: 0,093 cal/gºC; Ce Sn: 0,060 cal/gºC
URGENT

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, hdjsjfjruejchhehd
Define dew point. i am writing this part to be able to ask the question
Answers: 1



Chemistry, 23.06.2019 07:00, SMURFETTE86
Under what conditions will a gas be most likely to exhibit the ideal gas properties predicted by the ideal gas law? 1)high pressures and high temperature, because particles are forced closer together with higher kinetic energy, so intermolecular forces of attraction are weaker 2)high pressure and low temperature, because particles are forced closer together and moving slower, so the volume of the particles is less significant 3) low pressure and high temperature, because particles are spread farther apart and moving faster, so the intermolecular forces of attraction are weaker 4)low pressure and low temperature, because particles are spread farther apart with lower kinetic energy, so the volume of the particles is less significant
Answers: 2
Do you know the correct answer?
Para formar bronce, se mezclan 150g de cobre a 1100°C y 35g de estaño a 560°C. Determine la temperat...
Questions in other subjects:


Mathematics, 05.10.2019 14:30



Mathematics, 05.10.2019 14:30

Engineering, 05.10.2019 14:30

History, 05.10.2019 14:30

History, 05.10.2019 14:30



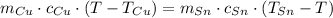
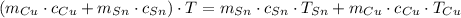
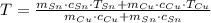 (1)
(1)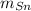 - Masa del estaño, en gramos.
- Masa del estaño, en gramos.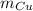 - Masa del cobre, en gramos.
- Masa del cobre, en gramos.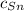 - Calor específico del estaño, en calorías por gramo-grados Celsius.
- Calor específico del estaño, en calorías por gramo-grados Celsius.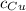 - Calor específico del cobre, en calorías por gramo-grados Celsius.
- Calor específico del cobre, en calorías por gramo-grados Celsius.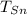 - Temperatura inicial del estaño, en grados Celsius.
- Temperatura inicial del estaño, en grados Celsius.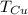 - Temperatura inicial del cobre, en grados Celsius.
- Temperatura inicial del cobre, en grados Celsius.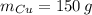 ,
, 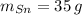 ,
, 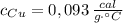 ,
, 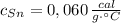 ,
, 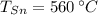 y
y 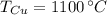 , entonces la temperatura final del sistema es:
, entonces la temperatura final del sistema es: