Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:40, caleb19moody
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2



Chemistry, 23.06.2019 03:10, 3jazybraxy
Which is true according to the law of conservation of energy
Answers: 1
Do you know the correct answer?
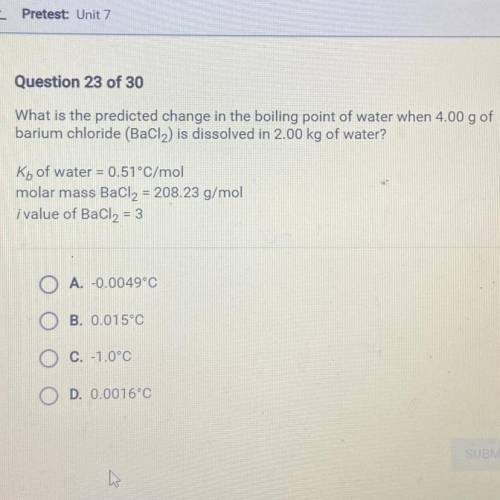
What is the predicted change in the boiling point of water when 4.00 g of
barium chloride (BaCl2) i...
Questions in other subjects:

Mathematics, 03.04.2020 00:38

Mathematics, 03.04.2020 00:38

Spanish, 03.04.2020 00:38



Mathematics, 03.04.2020 00:38