Answers: 3
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:30, AptAlbatross
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3

Chemistry, 23.06.2019 08:40, Riplilpeep
Which statement is true according to the kinetic theory? a. molecules of different gases with the same mass and temperature always have the same average density. b. molecules of different gases with the same mass and temperature always have the same average volume. c. molecules of different gases with the same mass and temperature always have the same pressure. d. molecules of different gases with the same mass and temperature always have the same molecular mass. e. molecules of different gases with the same mass and temperature always have the same average kinetic energy.
Answers: 1

Chemistry, 23.06.2019 13:00, journeyburks07
The gram molecular mass or co2 is the same as the gram molecular mass of
Answers: 2
Do you know the correct answer?
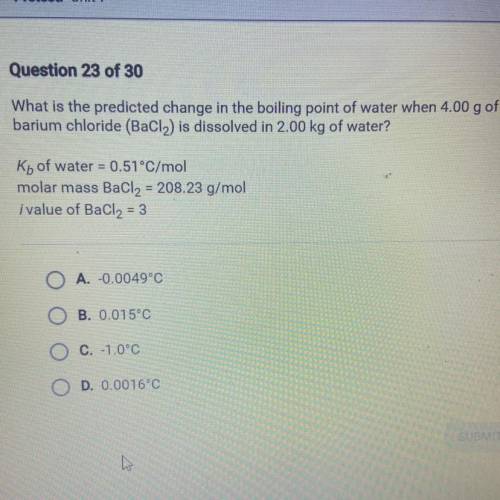
What is the predicted change in the boiling point of water when 4.00 g of
barium chloride (BaCl2) i...
Questions in other subjects:

Mathematics, 04.11.2020 20:20


English, 04.11.2020 20:20

Spanish, 04.11.2020 20:20

Chemistry, 04.11.2020 20:20


History, 04.11.2020 20:20



History, 04.11.2020 20:20