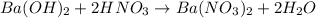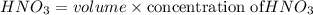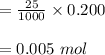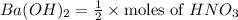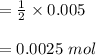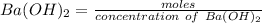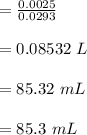Answer the following questions: (Questions about titration)
a. Why is it important to keep the NaOH solution covered at all times?
b. How will the molarity of NaOH solution be affected by its continued exposure to the atmosphere?
c. The pale pink color of the titration solution at the end point will fade to colorless after several minutes when exposed to the atmosphere. Account for this color change.
d. What volume (in mL) of 0.293 M Ba(OH)2 is required to neutralize 25.00 mL of 0.200M HNO3?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 09:00, floressavanna15
What properties would have caused early researchers to name hydrogen "inflammable air”
Answers: 3

Chemistry, 23.06.2019 11:50, halllawson
What is the oxidation half-reaction for this unbalanced redox equation? cr2o72– + fe2+ → cr3+ + fe3+ cr3+ → cr2o72– cr2o72– → cr3+ fe3+ → fe2+ fe2+ → fe3+?
Answers: 2

Chemistry, 23.06.2019 13:30, Willywill15
Did you mention that adding weight increased the pressure
Answers: 2

Chemistry, 23.06.2019 17:30, amazingmariah1234
Which statement correctly compares a law and a theory
Answers: 1
Do you know the correct answer?
Answer the following questions: (Questions about titration)
a. Why is it important to keep the NaOH...
Questions in other subjects:


Mathematics, 10.12.2019 16:31

History, 10.12.2019 16:31

Mathematics, 10.12.2019 16:31

Mathematics, 10.12.2019 16:31


Geography, 10.12.2019 16:31


Mathematics, 10.12.2019 16:31


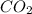 through dissolving in the solution and make carbonic acid
through dissolving in the solution and make carbonic acid 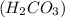 which reacts with the
which reacts with the 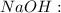
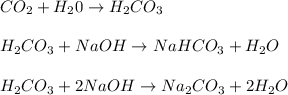
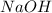 reacts with the dissolved
reacts with the dissolved 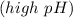 and colorless throughout the acidic solution
and colorless throughout the acidic solution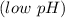 .
.
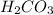 that gives the
that gives the 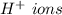
 lower pH.
lower pH.