(a). Ca has a larger ionization energy than 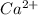 - False
- False
(b). Ar has a more exothermic electron affinity than Cl - False
(c). Ba has a larger ionization energy than 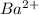 - False
- False
(d). Br is smaller than 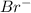 - True
- True
(e). 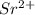 is larger than Sr - False
is larger than Sr - False
Explanation:
The amount of energy required in order to remove the most loosely bound electron from an atom or ion is called ionization energy.
An atom with large size will have low ionization energy.
The size of Ca is more as compared to 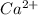 ion. Hence, Ca atom will have less ionization energy than
ion. Hence, Ca atom will have less ionization energy than 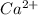 ion.
ion.
Similarly, Ba atom has less ionization energy than 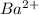 ion.
ion.
When an electron is added to an atom then the amount of energy released is called electron affinity.
Ar is a noble gas and its octet is already complete. So, it has less exothermic electron affinity than Cl because Cl needs one more electron to complete its octet.
When an element gain electrons then it acquires a negative charge and it is called an anion. The size of an anion is always larger than its parent atom.
For example, Br is smaller than 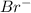 .
.
When an element lose electrons then it acquires a positive charge and it is called a cation. The size of a cation is always smaller than its parent atom.
For example, 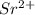 is smaller than Sr.
is smaller than Sr.
Thus, we can conclude that
(a). Ca has a larger ionization energy than 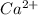 - False
- False
(b). Ar has a more exothermic electron affinity than Cl - False
(c). Ba has a larger ionization energy than 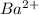 - False
- False
(d). Br is smaller than 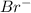 - True
- True
(e). 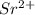 is larger than Sr - False
is larger than Sr - False


















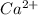 - False
- False
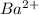 - False
- False
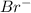 - True
- True
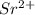 is larger than Sr - False
is larger than Sr - False



