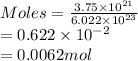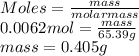Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:10, carsonjohnsonn
Can smoke be transformed into liquid or used as energy or both?
Answers: 2


Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 20:00, bbyjean9974
State one important difference between a physical change and a chemical change?
Answers: 1
Do you know the correct answer?
Determine the mass in grams of 3.75 x 10^21 atoms of zinc. (the mass of one mole of zinc is 65.39 g)...
Questions in other subjects:

Mathematics, 16.10.2020 08:01





Health, 16.10.2020 08:01


Mathematics, 16.10.2020 08:01


Mathematics, 16.10.2020 08:01


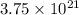 atoms of zinc is 0.405 g.
atoms of zinc is 0.405 g.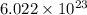 atoms. So, the number of moles in given number of atoms is as follows.
atoms. So, the number of moles in given number of atoms is as follows.