
Chemistry, 02.07.2021 05:40, httpcindy14
Using the balanced equation for the combustion of ethane: 2C2H6 + 7O2 → 4CO2 + 6H2O, how many moles of O2 needed to produce 12 moles of H2O?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:10, dookiefadep5n1tt
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 18:40, johnnysteeler9934
What is one real world example of a colligative property?
Answers: 2

Chemistry, 22.06.2019 19:00, innocentman69
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 22.06.2019 23:00, chastineondre7979
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1
Do you know the correct answer?
Using the balanced equation for the combustion of ethane: 2C2H6 + 7O2 → 4CO2 + 6H2O, how many moles...
Questions in other subjects:



Physics, 14.04.2020 01:58

History, 14.04.2020 01:59


Computers and Technology, 14.04.2020 01:59

Mathematics, 14.04.2020 01:59

Chemistry, 14.04.2020 01:59



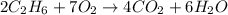
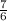 moles
moles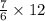 moles
moles moles
moles




