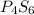Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, giusto1894
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2


Chemistry, 23.06.2019 00:00, scottykinkade7860
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1
Do you know the correct answer?
Compound X has a molar mass of 316.25 g*mol^-1 and the following composition:
element & mass %<...
Questions in other subjects:

Social Studies, 04.08.2019 02:00

History, 04.08.2019 02:00


Arts, 04.08.2019 02:00

Advanced Placement (AP), 04.08.2019 02:00

Biology, 04.08.2019 02:00

Social Studies, 04.08.2019 02:00

Chemistry, 04.08.2019 02:00

Business, 04.08.2019 02:00