
Chemistry, 02.07.2021 02:50, beccaxhope
35 POINTS - WILL GIVE BRAINLIEST!
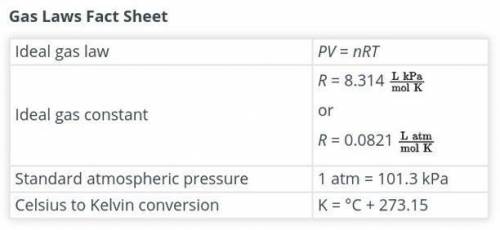
One of the main components of an airbag is the gas that fills it. As part of the design process, you need to
determine the exact amount of nitrogen that should be produced. Calculate the number of moles of
nitrogen required to fill the airbag. Show your work. Assume that the nitrogen produced by the chemical
reaction is at a temperature of 495°C and that nitrogen gas behaves like an ideal gas. Use this fact sheet
e to review the ideal gas law.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, ruleolivas
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 04:00, armahoney8566
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 06:00, tddreviews
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 09:20, kevinhernandez582
What will most likely happen when two bromine atoms bond together?
Answers: 3
Do you know the correct answer?
35 POINTS - WILL GIVE BRAINLIEST!
One of the main components of an airbag is the gas that fills it....
Questions in other subjects:


Mathematics, 24.05.2021 02:00


History, 24.05.2021 02:00

English, 24.05.2021 02:00










