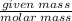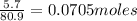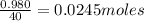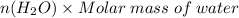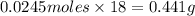Aqueous hydrobromic acid (HBr) will react with solid sodium hydroxide (NaOH) to produce aqueous sodium bromide (NaBr) and liquid water (H2O). Suppose 5.7 g of hydrobromic acid is mixed with 0.980 g of sodium hydroxide. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to significant digits.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, webbhlharryteach
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 03:30, electrofy456
What diagram shows the ionic compound of magnesium oxide
Answers: 2

Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 21:30, crystalbyrd79p8imrx
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3
Do you know the correct answer?
Aqueous hydrobromic acid (HBr) will react with solid sodium hydroxide (NaOH) to produce aqueous sodi...
Questions in other subjects:

Mathematics, 11.09.2020 17:01

Mathematics, 11.09.2020 17:01

Mathematics, 11.09.2020 17:01

Mathematics, 11.09.2020 17:01

Mathematics, 11.09.2020 17:01

Mathematics, 11.09.2020 17:01

Mathematics, 11.09.2020 17:01

Mathematics, 11.09.2020 17:01

Mathematics, 11.09.2020 17:01

Mathematics, 11.09.2020 17:01