Chemistry, 01.07.2021 16:00, ErrorNameTaken505
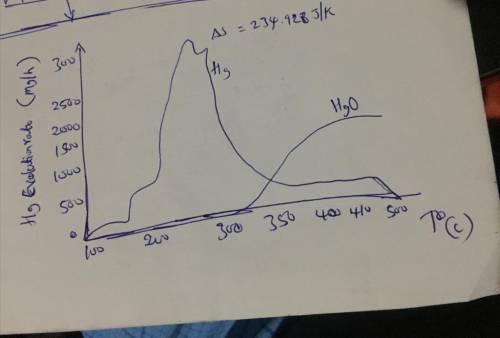
1. Show that heat flows spontaneously from high temperature to low temperature in any isolated system (hint: use entropy change that occurs during the process for your proof). 2. Work out the entropy change for the decomposition of mercuric oxide using mathematical and graphical arguments.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:10, PineaPPle663
Nuclear fusion is the source of energy for stars. besides hydrogen, which other element is most likely also common in stars?
Answers: 1

Chemistry, 22.06.2019 05:30, fgcherubin
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 11:10, hannah2757
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 12:30, kaliyab191
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2
Do you know the correct answer?
1. Show that heat flows spontaneously from high temperature to low temperature in any isolated syste...
Questions in other subjects:


Mathematics, 18.08.2019 22:00

Mathematics, 18.08.2019 22:00

English, 18.08.2019 22:00



Mathematics, 18.08.2019 22:00

Mathematics, 18.08.2019 22:00

Mathematics, 18.08.2019 22:00

Physics, 18.08.2019 22:00