
Chemistry, 30.06.2021 03:00, ashtonrieper1132
En la fermentación del alcohol, la levadura convierte la glucosa en etanol y dióxido de carbono:
C6H12O6(s) → 2C2H5OH(l) + 2CO2(g)
Si reaccionan 5.97 g de glucosa y se recolectan 1.44 L de CO2 gaseoso, a 293 K y 0.984 atm, ¿cuál
es el rendimiento porcentual de la reacción

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, baileysosmart
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3


Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2

Do you know the correct answer?
En la fermentación del alcohol, la levadura convierte la glucosa en etanol y dióxido de carbono:
C6...
Questions in other subjects:




Mathematics, 05.02.2021 04:50

Spanish, 05.02.2021 04:50

Mathematics, 05.02.2021 04:50


Mathematics, 05.02.2021 04:50

Business, 05.02.2021 04:50


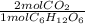 = 0.0664 mol CO₂
= 0.0664 mol CO₂



