
Chemistry, 29.06.2021 21:40, karmaxnagisa20
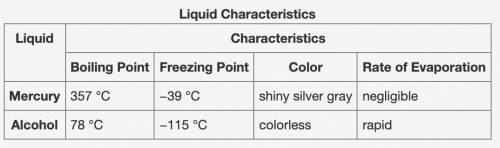
A student collected the data shown in the table below during an experiment.
Based on the data, which of the following conclusions can be made about the use of mercury and alcohol thermometers?
A - An alcohol thermometer can measure the freezing point of a liquid that freezes at −80 °C.
B - An alcohol thermometer can measure a wider range of temperatures in a laboratory.
C - An alcohol thermometer is more reliable to measure the temperature of a liquid in a beaker that is 80 °C.
D - An alcohol thermometer is better to measure the boiling points of colorless liquids.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, shreyapatel2004
How many atoms of oxygen are contained in 160 grams of n2o3
Answers: 2



Chemistry, 22.06.2019 18:20, juansebas35
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
Do you know the correct answer?
A student collected the data shown in the table below during an experiment.
Based on the data, whic...
Questions in other subjects:

History, 07.06.2021 19:30






French, 07.06.2021 19:30

Mathematics, 07.06.2021 19:30

Mathematics, 07.06.2021 19:30

Mathematics, 07.06.2021 19:30






