
Chemistry, 28.06.2021 20:50, marlenemedina247
Calculate [H3O+] and [OH−] for each of the following solutions at 25 ∘C given the pH.
pH= 2.89

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, omoaye
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 23.06.2019 06:00, mirzakasumovic8926
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2

Chemistry, 23.06.2019 11:00, lemonsalt9378
The decimals you found in part b are called repeating decimals. in the gizmo, repeating decimals are rounded to two places. how does the gizmo show you that a decimal has been rounded?
Answers: 3
Do you know the correct answer?
Calculate [H3O+] and [OH−] for each of the following solutions at 25 ∘C given the pH.
pH= 2.89...
pH= 2.89...
Questions in other subjects:

Mathematics, 27.09.2019 07:10

English, 27.09.2019 07:10



English, 27.09.2019 07:10


Mathematics, 27.09.2019 07:10

English, 27.09.2019 07:10

History, 27.09.2019 07:10


![[H_{3}O^{+}]](/tpl/images/1385/4271/71b58.png) is 0.0012 M and
is 0.0012 M and ![[OH^{-}]](/tpl/images/1385/4271/e46dd.png) is
is 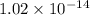 .
. ![pH = - log [H^{+}]\\2.89 = - log [H^{+}]\\conc. H^{+} = 0.0012 M](/tpl/images/1385/4271/e5499.png)
![pOH = - log [OH^{-}]\\13.99 = - log [OH^{-}]\\conc. OH^{-} = 1.02 \times 10^{-14} M](/tpl/images/1385/4271/f05e1.png)




