
Chemistry, 25.06.2021 14:00, maggie9459
A mixture of reactants and products for the reacting shown below is at equilibrium 2.0 L container. What would most likely happen to the equilibrium if the volume of the container were increased to 4.0 L?
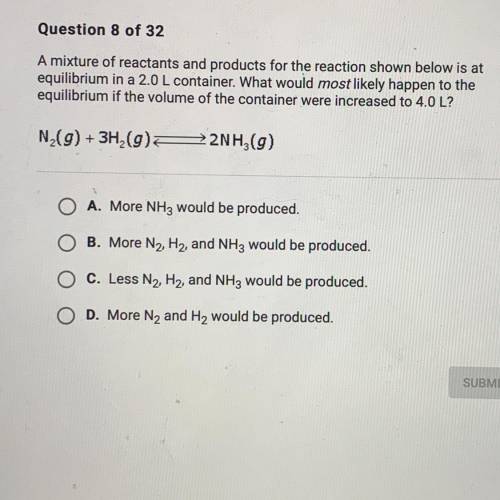

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:00, HaydenSturgis1
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 20:00, batoolishak7475
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 22.06.2019 23:30, sweaversw9602
What are the similarities between compounds and mixtures?
Answers: 3

Chemistry, 23.06.2019 04:31, diamondscott9297
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1
Do you know the correct answer?
A mixture of reactants and products for the reacting shown below is at equilibrium 2.0 L container....
Questions in other subjects:

Mathematics, 14.12.2021 05:00


History, 14.12.2021 05:00

Mathematics, 14.12.2021 05:00


Advanced Placement (AP), 14.12.2021 05:00

Mathematics, 14.12.2021 05:00

Mathematics, 14.12.2021 05:00


English, 14.12.2021 05:00






