
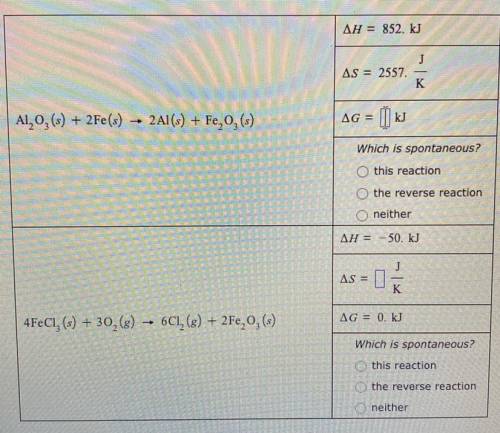
A chemical engineer is studying the two reactions shown in the table below.
In each case, she fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 57.0°C and constant total pressure. Then, she measures that reaction enthalpy delta H and the reaction entropy delta S of the first reaction, and the reaction enthalpy delta H and reaction free energy delta G of the second reaction. The results of her measurements are shown in the table.
Complete the table. That is, calculate delta G for the first reaction and delta S for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward or reverse reaction is spontaneous because the system is at equilibrium.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:30, logan12345677885675
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2


Chemistry, 22.06.2019 14:50, alexabbarker9781
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1
Do you know the correct answer?
A chemical engineer is studying the two reactions shown in the table below.
In each case, she fills...
Questions in other subjects:




History, 09.03.2021 07:50

Mathematics, 09.03.2021 07:50


Health, 09.03.2021 07:50










