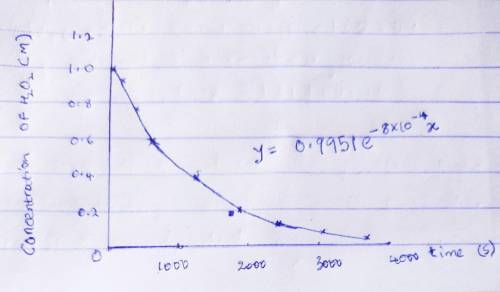
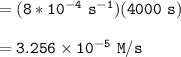The decomposition of hydrogen peroxide was studied, and the following data were obtained at a particular temperature. Time (s)[H2O2] (mol/L)0 1.00120 ± 10.91300 ± 10.78600 ± 10.591200 ± 10.371800 ± 10.222400 ± 10.133000 ± 10.0823600 ± 10.050Assuming that the rate= -delta [H2O2]/delta t determine the rate law, integrated rate law, and the value of the rate constant. Calculate [H2O2] at 4000. s after the start of the reaction.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, shadekashakay
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 03:00, cheesecake1919
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 04:30, salvadorperez26
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2
Do you know the correct answer?
The decomposition of hydrogen peroxide was studied, and the following data were obtained at a partic...
Questions in other subjects:



English, 05.12.2020 01:00



Mathematics, 05.12.2020 01:00

English, 05.12.2020 01:00

English, 05.12.2020 01:00

Social Studies, 05.12.2020 01:00


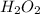 which declines exponentially in relation to the time and it obeys the equation:
which declines exponentially in relation to the time and it obeys the equation: 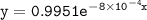
![\mathtt{ [A] = [A]_o e^{-kt}}](/tpl/images/1383/5053/858f7.png)
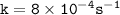
![\mathtt{= k[H_2O_2]}](/tpl/images/1383/5053/b66a7.png)
![\mathtt{[H_2O_2] = [H_2O_2]_oe^{-(8*10^{-4})t}}](/tpl/images/1383/5053/3f9ba.png)
![\mathtt{[H_2O_2] = [H_2O_2]_oe^{-8*10^{-4}(t)}}](/tpl/images/1383/5053/adf3d.png)
![\mathtt{[H_2O_2] = (1.00\ M)*e^{-8*10^{-4}(4000)\ s}}](/tpl/images/1383/5053/a221b.png)
![\mathtt{[H_2O_2] =0.0407 \ M}](/tpl/images/1383/5053/c14b9.png)