Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, youngchapo813p8d9u1
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 21:30, Turtlelover05
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 22.06.2019 23:00, SophieCasey
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
Do you know the correct answer?
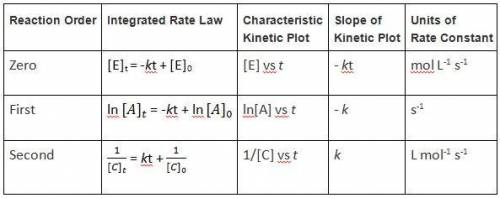
The rate of the reaction is 1.6*10-2 M/s when the concentration of A is 0.15 M. Calculate the rate c...
Questions in other subjects:


Social Studies, 16.09.2019 22:30

Mathematics, 16.09.2019 22:30




Mathematics, 16.09.2019 22:30

Mathematics, 16.09.2019 22:30



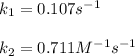
![r=k[A]\\\\r=k[A]^2](/tpl/images/1383/1866/b314f.png)
![k=\frac{r}{[A]}=\frac{1.6x10^{-2}M/s}{0.15M}=0.107s^{-1} \\\\k=\frac{r}{[A]^2}=\frac{1.6x10^{-2}M/s}{(0.15M)^2}=0.711M^{-1}s^{-1}](/tpl/images/1383/1866/c6aca.png)