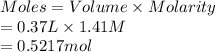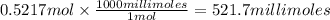Chemistry, 24.06.2021 15:50, brooklynpage5283
A chemist adds 370.0mL of a 1.41/molL potassium iodide KI solution to a reaction flask. Calculate the millimoles of potassium iodide the chemist has added to the flask. Be sure your answer has the correct number of significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:00, teacherpreacher
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 23.06.2019 03:30, cupcake3103670
Name 3 types of energy you see being used as you look around a classroom
Answers: 1

Do you know the correct answer?
A chemist adds 370.0mL of a 1.41/molL potassium iodide KI solution to a reaction flask. Calculate th...
Questions in other subjects:

Mathematics, 04.06.2020 15:00


Mathematics, 04.06.2020 15:00


Mathematics, 04.06.2020 15:00


Mathematics, 04.06.2020 15:00

Mathematics, 04.06.2020 15:00

Mathematics, 04.06.2020 15:00

Mathematics, 04.06.2020 15:00