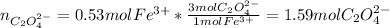Chemistry, 23.06.2021 07:40, smithmalyk4
Kc is 1.67 x 10^20 at 25 °C for the formation of iron(III) oxalate complex ion:
Fe^3+ (aq) + 3C2O4^2- (aq) <--> [Fe(C204)3]^3- (aq)
Determine the number of moles of C2O4^2- used to react with 0.53 moles of Fe^3+.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, aeverettpdzrvo
The most efficient way to establish the best possible economizer position is to measure
Answers: 1


Chemistry, 22.06.2019 00:30, TMeansStupidity
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1
Do you know the correct answer?
Kc is 1.67 x 10^20 at 25 °C for the formation of iron(III) oxalate complex ion:
Fe^3+ (aq) + 3C2O4^...
Questions in other subjects:

History, 16.08.2020 01:01










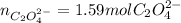
![Kc=\frac{[[Fe(C_2O_4)_3]^{3-}]}{[Fe^{3+}][C_2O_4^{2-}]^3}](/tpl/images/1382/0865/5bd65.png)