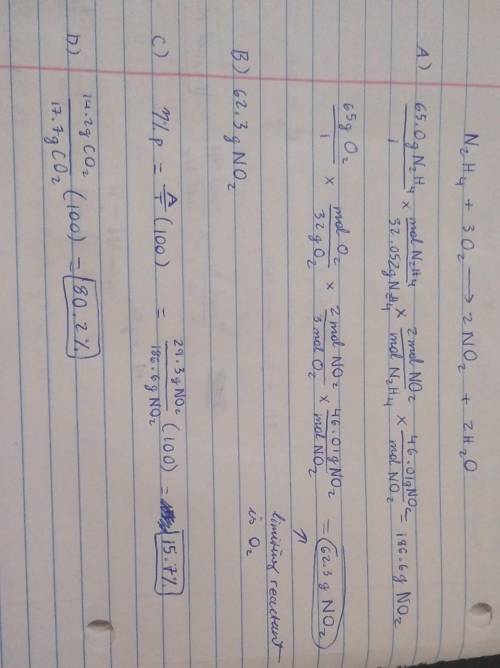19) Hydrazine, N2H4, a substance used as rocket fuel, reacts with oxygen as follows:
N2H4 (1) + 3 O2(g) → 2 NO2(g) + 2 H20 (8)
A) If 65.0 g of hydrazine are reacted with 65.0 g of oxygen, which is the limiting reactant?
usg of Oz X Imol
.
B) How many grams of NO2 are produced from the limiting reactant in part A?
C) If 29.3 g of NO2 are obtained from the reaction in Part A, what is the percent yield?
20) What is the percent yield if 14.2 g of CO2 were produced and 17.7 g were calculated to be
produced from the reaction?

Answers: 2
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
Do you know the correct answer?
19) Hydrazine, N2H4, a substance used as rocket fuel, reacts with oxygen as follows:
N2H4 (1) + 3 O...
Questions in other subjects:

Mathematics, 19.12.2019 08:31

Physics, 19.12.2019 08:31

Biology, 19.12.2019 08:31


History, 19.12.2019 08:31



History, 19.12.2019 08:31


Mathematics, 19.12.2019 08:31