
Chemistry, 22.06.2021 04:40, bearminar2156
2 NH3 + 3 CuO --> 3 Cu + N2 + 3 H2O
In the above equation, how many moles of N2 can be made when 113.6 grams of CuO are consumed?
Round your answer to the nearest tenth. If your answer is a whole number like 4, report the answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect. :
Element Molar Mass
Hydrogen
1
Nitrogen
14
Copper
63.5
Oxygen
16

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, mosthatedpicky1
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2


Chemistry, 23.06.2019 00:50, alainacorkell6472
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1
Do you know the correct answer?
2 NH3 + 3 CuO --> 3 Cu + N2 + 3 H2O
In the above equation, how many moles of N2 can be made when...
Questions in other subjects:


Physics, 09.10.2021 01:00

Mathematics, 09.10.2021 01:00

Physics, 09.10.2021 01:00

History, 09.10.2021 01:00






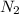 are produced in the reaction
are produced in the reaction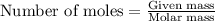 ......(1)
......(1)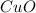 = 113.6 g
= 113.6 g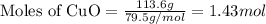
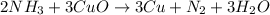
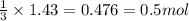 of
of 



