gas

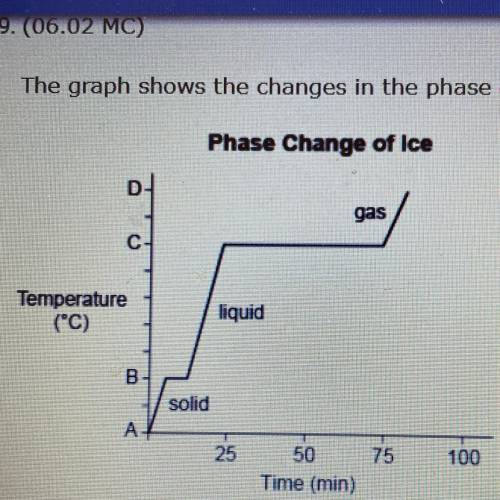
The graph shows the changes in the phase of ice when it is heated.
Phase Change of Ice
gas
C
Temperature
(C)
liquid
в.
solid
A-
25
50
75
100
Time (min)
Which of the following temperatures describes the value of A? (5 points)
0 °C, because it is the melting point gf ice.
O 0 °C, because it is the freezing point of water.
Less than 0 °C, because B represents the temperature at which ice melts.
Less than 0 °C, because B represents the temperature at which water evaporates.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:20, Jessicadiaz8602
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 12:10, coastieltp58aeg
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 13:30, annanikherrera
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 15:00, raeprince9213
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
Do you know the correct answer?
The graph shows the changes in the phase of ice when it is heated.
Phase Change of Ice
gas
gas
Questions in other subjects:

Mathematics, 14.01.2021 20:10



Mathematics, 14.01.2021 20:10


Physics, 14.01.2021 20:10


History, 14.01.2021 20:10


Arts, 14.01.2021 20:10






