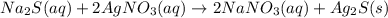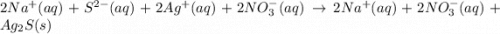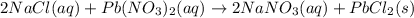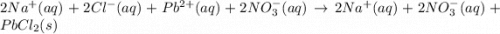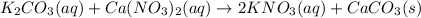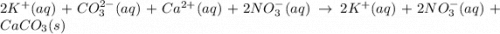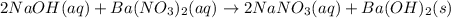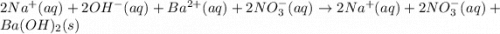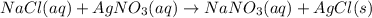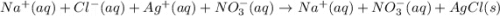Chemistry, 21.06.2021 18:30, jjsavage06
When the following aqueous solutions are mixed together, a precipitate forms. Balance the net ionic equation in standard form for the reaction that occurs and determine the sum of the coefficients.
Sodium sulfide and silver nitrate - 3 or 4
Lead(II) nitrate and sodium chloride -3 or 4
Calcium nitrate and potassium carbonate - 3or 4
Barium nitrate and sodium hydroxide -3 or 4
Silver nitrate and sodium chloride -3 or 4

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, mauifrifer3986
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1


Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 19:00, Jasoncookies23
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
Do you know the correct answer?
When the following aqueous solutions are mixed together, a precipitate forms. Balance the net ionic...
Questions in other subjects:

Biology, 20.10.2021 18:00




Mathematics, 20.10.2021 18:00

Physics, 20.10.2021 18:00


Mathematics, 20.10.2021 18:00

English, 20.10.2021 18:00


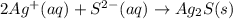 and the sum of coefficients is 4
and the sum of coefficients is 4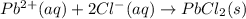 and the sum of coefficients is 4
and the sum of coefficients is 4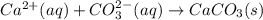 and the sum of coefficients is
and the sum of coefficients is 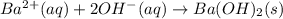 and the sum of coefficients is 4
and the sum of coefficients is 4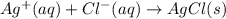 and the sum of coefficients is 3
and the sum of coefficients is 3