
249 g of potassium iodide, KI, is mixed with 496.5 g of lead(II) nitrate, Pb(NO3)2.
The equation of the reaction is represented below.
[Pb(NO3)2 = 331; KI = 166; PbI2 = 461, KNO3 = 101]
Calculate the number of moles of excess reagent left.
Give your answer to three significant figures.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, homeschool0123
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 10:40, rntaran2002
What is the ph of a 0.0010 m hno3? 1.0 3.0 4.0 5.0
Answers: 2

Chemistry, 22.06.2019 20:00, ahnorthcutt4965
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1
Do you know the correct answer?
249 g of potassium iodide, KI, is mixed with 496.5 g of lead(II) nitrate, Pb(NO3)2.
The equation of...
Questions in other subjects:

Mathematics, 04.02.2021 22:10

English, 04.02.2021 22:10



Mathematics, 04.02.2021 22:10


Computers and Technology, 04.02.2021 22:10




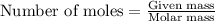 ......(1)
......(1) = 249 g
= 249 g
 :
: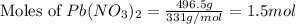

 of lead(II) nitrate
of lead(II) nitrate



