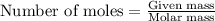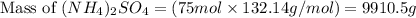Chemistry, 20.06.2021 14:00, amcdaniel1
Ammonium sulfate (NH4)2SO4 is made by reacting 25.0 L of 3.0 mol/L H2SO4 with 3.1× 103 L of NH3 at a pressure of 0.68 atm and a temperature of 298 K according to the following reaction .
NH3(g) + H2SO4(aq) → (NH4)2SO4 (aq)
How many grams of ammonium sulfate are produced?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, jewelz5887
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 10:10, ragegamer334p3xlso
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3


Chemistry, 22.06.2019 14:30, srutkowske1489
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1
Do you know the correct answer?
Ammonium sulfate (NH4)2SO4 is made by reacting 25.0 L of 3.0 mol/L H2SO4 with 3.1× 103 L of NH3 at a...
Questions in other subjects:

Mathematics, 14.07.2019 14:00





Mathematics, 14.07.2019 14:00

Biology, 14.07.2019 14:00


Biology, 14.07.2019 14:00


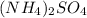 produced is 9910.5 g
produced is 9910.5 g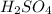 :
: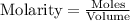 ......(1)
......(1) 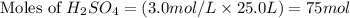
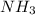 :
: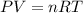 .......(2)
.......(2)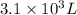
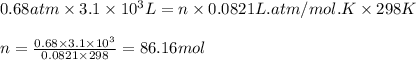
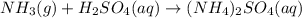
 of
of