
Gaseous ethane (CH, CH,) will react with gaseous oxygen (02) to produce gaseous carbon dioxide (CO2) and gaseous water (H,0). Suppose 4.21 g of
ethane is mixed with 31. 9 of oxygen. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. Be sure your answer has
the correct number of significant digits.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, madisonrosamond99
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2


Chemistry, 22.06.2019 18:10, ellemarshall13
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 22.06.2019 18:30, kate3887
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
Do you know the correct answer?
Gaseous ethane (CH, CH,) will react with gaseous oxygen (02) to produce gaseous carbon dioxide (CO2)...
Questions in other subjects:

Mathematics, 01.02.2021 17:00

Social Studies, 01.02.2021 17:00

Mathematics, 01.02.2021 17:00




Mathematics, 01.02.2021 17:00

Mathematics, 01.02.2021 17:00

English, 01.02.2021 17:00

Chemistry, 01.02.2021 17:00


 produced is 12.32 g
produced is 12.32 g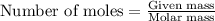 ......(1)
......(1)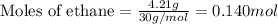

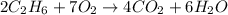
 of oxygen gas
of oxygen gas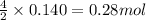 of
of 




