
Chemistry, 19.06.2021 06:10, floreschachi2873
Read the following reactions. Reaction 1: CaO(s) + CO2(g) → CaCO3(s) Reaction 2: H2O(g) → H2O(l) Which reaction leads to an increase in entropy? Only Reaction 1 Only Reaction 2 Both Reaction 1 and 2 Neither Reaction 1 nor 2

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, nothingworksoutforme
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 08:30, microwave13016
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 10:30, kylemartinez13
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Do you know the correct answer?
Read the following reactions. Reaction 1: CaO(s) + CO2(g) → CaCO3(s) Reaction 2: H2O(g) → H2O(l) Whi...
Questions in other subjects:



English, 08.04.2020 18:48

Mathematics, 08.04.2020 18:48





History, 08.04.2020 18:48


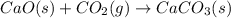 and it shows that product is in solid state. Therefore, entropy is decreasing.
and it shows that product is in solid state. Therefore, entropy is decreasing.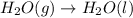 and it shows that gas is converted into liquid. Therefore, entropy is also decreasing here.
and it shows that gas is converted into liquid. Therefore, entropy is also decreasing here.



