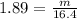Chemistry, 19.06.2021 05:30, thegreentnt5025
A 16.4L sample of NO2(S) has a density of 1.89 g/L. What is the mass of the sample NO2(s)?
A) 31.0 grams
B) 14.5 grams
C) 8.68 grams
D) 0.115 grams

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, lakenyagillard79
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 03:00, parisaidan366
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 03:00, bobbycisar1205
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 05:30, livigrace9004
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2
Do you know the correct answer?
A 16.4L sample of NO2(S) has a density of 1.89 g/L. What is the mass of the sample NO2(s)?
A) 31.0...
Questions in other subjects:











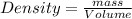 , or
, or 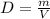 .
.