
Chemistry, 18.06.2021 22:00, davisearron
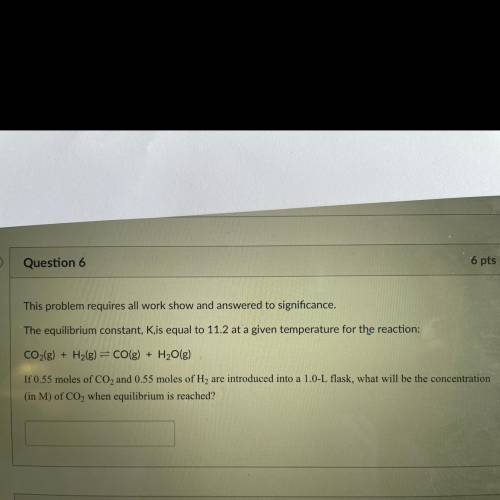
This problem requires all work show and answered to significance.
The equilibrium constant, Kis equal to 11.2 at a given temperature for the reaction:
CO2(g) + H2(g) = CO(g) + H2O(g)
If 0.55 moles of CO2 and 0.55 moles of H2 are introduced into a 1.0-L flask, what will be the concentration
(in M) of CO2 when equilibrium is reached?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, erikloza12pdidtx
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 05:30, nuclearfire278
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease. correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1


Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1
Do you know the correct answer?
This problem requires all work show and answered to significance.
The equilibrium constant, Kis equ...
Questions in other subjects:

Mathematics, 20.04.2020 20:03


Mathematics, 20.04.2020 20:03




Mathematics, 20.04.2020 20:03

Advanced Placement (AP), 20.04.2020 20:03

Mathematics, 20.04.2020 20:03






