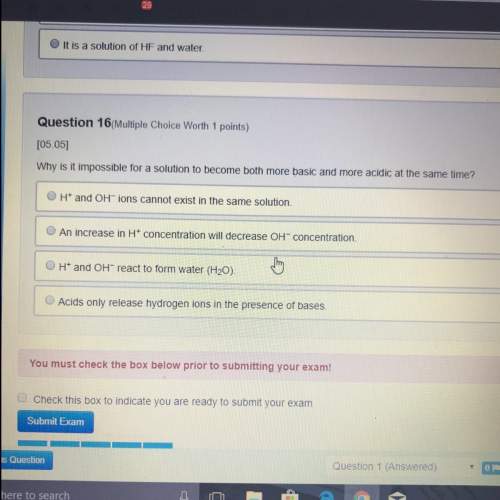
Chemistry, 18.06.2021 20:00, kassandrarosario1115
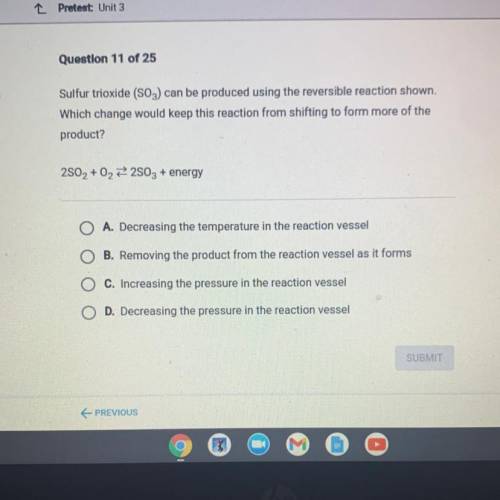
Sulfur trioxide (SO3) can be produced using the reversible reaction shown.
Which change would keep this reaction from shifting to form more of the
product?
2802 + 02 22803 + energy
A. Decreasing the temperature in the reaction vessel
B. Removing the product from the reaction vessel as it forms
C. Increasing the pressure in the reaction vessel
D. Decreasing the pressure in the reaction vessel

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 22:00, aliciaa101
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Chemistry, 22.06.2019 22:30, eduardoguizar8787
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
Do you know the correct answer?
Sulfur trioxide (SO3) can be produced using the reversible reaction shown.
Which change would keep...
Questions in other subjects:




Mathematics, 31.01.2022 23:10

Mathematics, 31.01.2022 23:10




Health, 31.01.2022 23:10