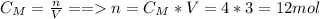You have 4 litres of a 3.0 mol/L solution of NaCl in a
chemical store room.
How many moles of...

Chemistry, 18.06.2021 17:40, donaji1024perez
You have 4 litres of a 3.0 mol/L solution of NaCl in a
chemical store room.
How many moles of NaCl are present? *
0.75 mol
1.33 mol
12 mol

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:10, vapelordcarl69
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3


Chemistry, 23.06.2019 00:20, HernanJe6
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Chemistry, 06.11.2019 18:31

Mathematics, 06.11.2019 18:31


Mathematics, 06.11.2019 18:31

History, 06.11.2019 18:31


History, 06.11.2019 18:31

History, 06.11.2019 18:31

Advanced Placement (AP), 06.11.2019 18:31