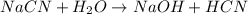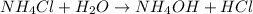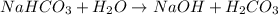Chemistry, 18.06.2021 02:00, alananicoleee
State whether 0.1 M solutions of each of the following salts are acidic, basic, or neutral. Explain your reasoning for each by writing a balanced net ionic equation to describe the behavior of each nonneutral salt in water: NaCN, KNO3, NH4Cl, NaHCO3, and Na3PO4.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:20, Jessicadiaz8602
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1


Chemistry, 23.06.2019 09:30, alexabdercmur
Sheela and her brother hari were sitting in the living room, watching tv. suddenly hari said that he thinks something is burning in the other room. how did he get the burning smell?
Answers: 3
Do you know the correct answer?
State whether 0.1 M solutions of each of the following salts are acidic, basic, or neutral. Explain...
Questions in other subjects:


History, 18.12.2020 15:10


Mathematics, 18.12.2020 15:10

English, 18.12.2020 15:10





Mathematics, 18.12.2020 15:10


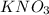 is a neutral salt
is a neutral salt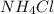 is an acidic salt
is an acidic salt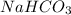 is a basic salt
is a basic salt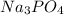 is a neutral salt
is a neutral salt