Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, daryondaniels28
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2



Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
Do you know the correct answer?
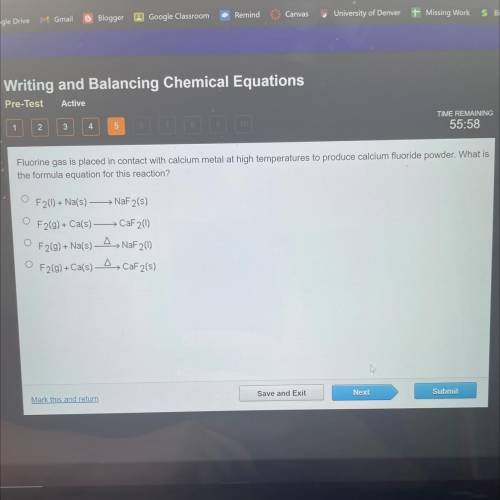
Fluorine gas is placed in contact with calcium metal at high temperatures to produce calcium fluorid...
Questions in other subjects:

History, 04.07.2019 21:00

Mathematics, 04.07.2019 21:00


Geography, 04.07.2019 21:00

Chemistry, 04.07.2019 21:00

Mathematics, 04.07.2019 21:00


Health, 04.07.2019 21:00

Mathematics, 04.07.2019 21:00

English, 04.07.2019 21:00