
Part C
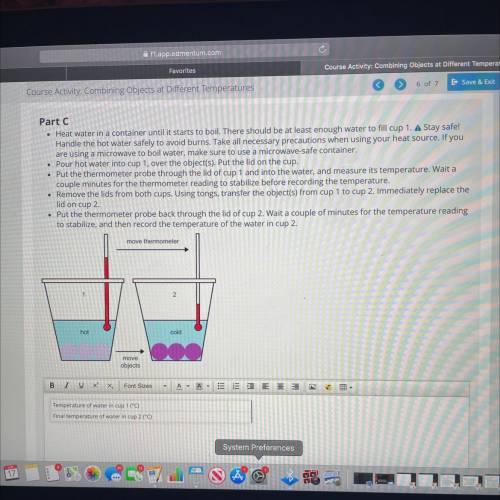
• Heat water in a container until it starts to boil. There should be at least enough water to fill cup 1. A Stay safe!
Handle the hot water safely to avoid burns. Take all necessary precautions when using your heat source. If you
are using a microwave to boil water, make sure to use a microwave-safe container.
• Pour hot water into cup 1, over the object(s). Put the lid on the cup.
• Put the thermometer probe through the lid of cup 1 and into the water, and measure its temperature. Wait a
couple minutes for the thermometer reading to stabilize before recording the temperature.
• Remove the lids from both cups. Using tongs, transfer the object(s) from cup 1 to cup 2. Immediately replace the
lid on cup 2
• Put the thermometer probe back through the lid of cup 2. Wait a couple of minutes for the temperature reading
to stabilize, and then record the temperature of the water in cup 2.
e
selected files
5
Comments
move thermometer
1
2
hot
cold
move
objects
B
IV X X
Font Sizes
- A - A - E 3
Temperature of water in cup 1 (C)
Final temperature of water in cup 2(0)
System Preferences

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2

Chemistry, 22.06.2019 09:00, mercymain1014
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 12:30, pup88
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2
Do you know the correct answer?
Part C
• Heat water in a container until it starts to boil. There should be at least enough water t...
Questions in other subjects:








Mathematics, 23.12.2020 21:10

Spanish, 23.12.2020 21:10






