
Chemistry, 17.06.2021 01:00, breemills9953
Calculate the volume of 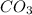 that will be produced from 0.5 moles of
that will be produced from 0.5 moles of 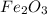 according to the equation below:
according to the equation below:
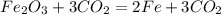
if one mole of gas occupies 22.4dm^3 , calculate the volume of 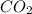 that will be produced.
that will be produced.
Question 2
Calculate the mass of impurity in a sample of 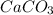 . iF 0.05 mol of
. iF 0.05 mol of 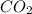 was formed when 7g of the sample reacts with excess dilute HCl . The equation for the reaction is :
was formed when 7g of the sample reacts with excess dilute HCl . The equation for the reaction is :
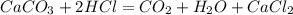

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:20, michellectucker1982
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1


Chemistry, 23.06.2019 09:50, jay4881
T(s) in2os] (m) 0 185 2.39 546 1.90 725 1.70 the decomposition of n205 can be described by the equation 2.68 given these data for the reaction at 45°c in carbon tetrachloride solution, calculate the average rate of reaction for each successive time interval. ntr s to 185 s 185 s to 546 s 546 s to 725 s number number number reaction rate: m/s m/s m/s
Answers: 1
Do you know the correct answer?
Calculate the volume of that will be produced from 0.5 moles of according to the equation below:...
Questions in other subjects:

Mathematics, 21.02.2020 05:31



Mathematics, 21.02.2020 05:31


Computers and Technology, 21.02.2020 05:31









